From the initial source CTIBIOTECH has experience of:
- Peripheral blood
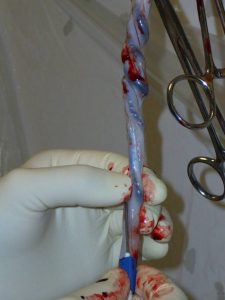
- Umbilical cord blood
- Placental tissues
- Umbilical cord wharton’s jelly
- Amnion
- Amniotic fluid
- Bone marrow
- Skin
- Adipose
Processing of tissues is an essential element in the production of viable and usable stem cells without contamination and unnecessary stimulation of the cells.
Processing of physical tissue is different from processing fluidic tissue (Eg., peripheral blood) and requires processes without heat or trauma to the tissue.
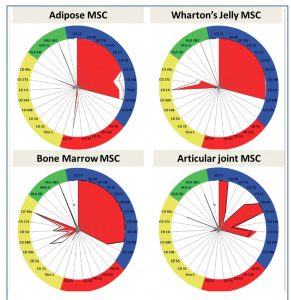
Harvested selected stem and progenitor cell populations require sophisticated examination from the point of harvest through to final use.
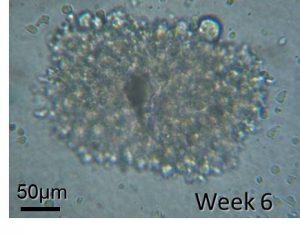
Such characterisation has allowed development of highly sophisticated techniques to harvest extremely rare cell populations such as the Cord Blood-derived Embryonic-like Cells (CBE)

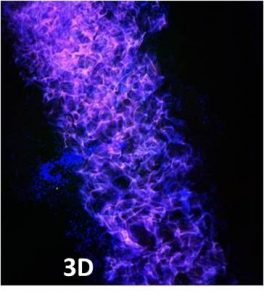
Rotating culture works to counteract natural gravity-based influences, such as basal adherence and allows cell networks to form which better resemble the human system.
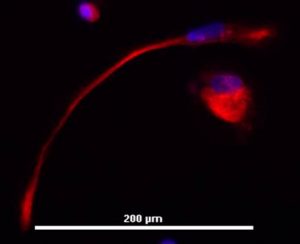
A particular speciality of our researchers is defined differentiation, particularly without animal products.
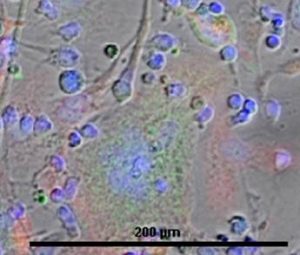
Cellular interactions between different cell types can therefore be controlled.
CTIBIOTECH staff have designed, created and implemented several GMP cell bioprocessing centres in both the academic and commercial sectors. These GMP centres have been used for both research and human clinical therapies.