- medical device developments, leading to the award of 2 CE marks
- medical device development, specific for the USA market, leading to 2 FDA awards already passed and an additional FDA award in review
- testing of biomaterials, leading to patented products
A case study appears below.
 Medical device for rapid processing of Human Cord Blood, using no electricity
Medical device for rapid processing of Human Cord Blood, using no electricity
![]() Contracting company: KANEKA Corporation, Osaka, Japan. A company with a 5 billion dollar turnover (2014).
Contracting company: KANEKA Corporation, Osaka, Japan. A company with a 5 billion dollar turnover (2014).

RESULT: CellEffic CB product for processing umbilical cord blood developed. Two successful CE marks awarded. Trademarks awareded globally. Work published in abstracts and 2 peer-reviewed journal publications. Product on sale.
First Journal publication from the work:
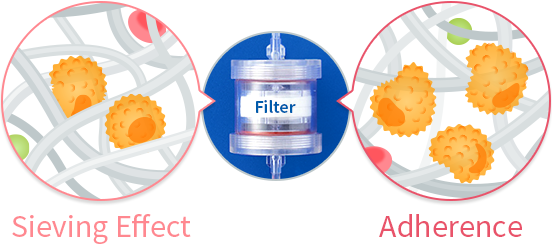
Introduction: Cord blood (CB) is being increasingly used as a source of hematopoietic stem cells for transplantation to treat diseases of the blood and immune systems, and there is an urgent need to expand CB banking worldwide. CB processing requires costly machinery or a clean room that hampers wider application of CBT particularly in the developing countries.
Methods: We developed a novel filtration system using a nonchemical-coated and nonwoven polyester fabric filter, which traps cells through affinity and does not require centrifugation or potentially toxic chemicals.
Results: Cell processing with the device resulted in minimum cell loss of total cells and CD34+cells, without impairing the ability of CD34+cells to engraft and differentiate both in vivo and in vitro.
Conclusion: CB processing with this device is simple, cost-effective, and nontoxic without requiring costly equipment will thus facilitate international CB banking, which helps in meeting the increasing worldwide demand for CB for allogeneic hematopoietic stem cell transplantation.