We live in a human world
Every day human tissues are thrown away after surgery. We save them and give them new purpose, To advance human therapies, to develop new medicines, to test cosmetics or test anything that comes into contact with us.
For a better human world
About us
CTIBIOTECH is a pioneering company based in Lyon (France), specializing in the development of advanced human tissues for biomedical research and the pharmaceutical industry, and for future therapies. Founded in 2009, CTIBIOTECH’s revolutionizes research and development of new treatments by providing alternative solutions to animal testing with humanized systems.
At CTIBIOTECH, we believe in innovation for a healthier future. Our goal is to create accurate and reliable human tissues that enable researchers and companies to better understand diseases, test drugs, and develop new treatments. We use cutting-edge technologies, such as stem cells and 3D printing, to create 3D tissue and cell models that realistically simulate the structure and function of human tissues.
Our Mission
Explore our industries at CTIBIOTECH


Skin tissues innovation
and testing
Award winning skin tests and the latest skin models for testing cosmetics, drugs and devices
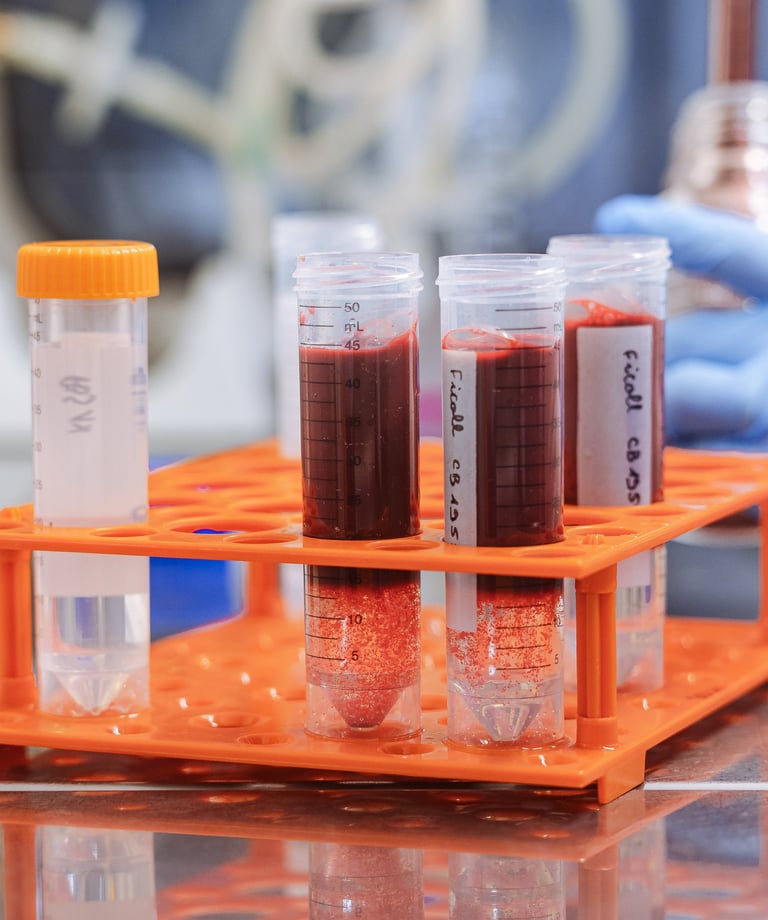
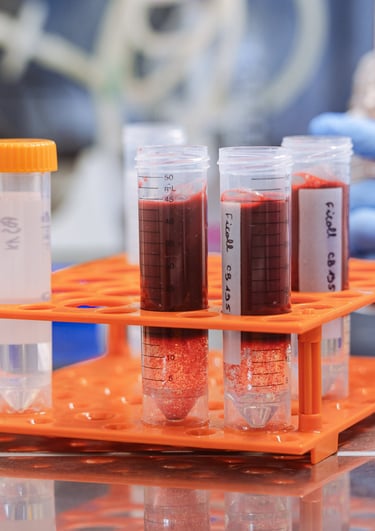
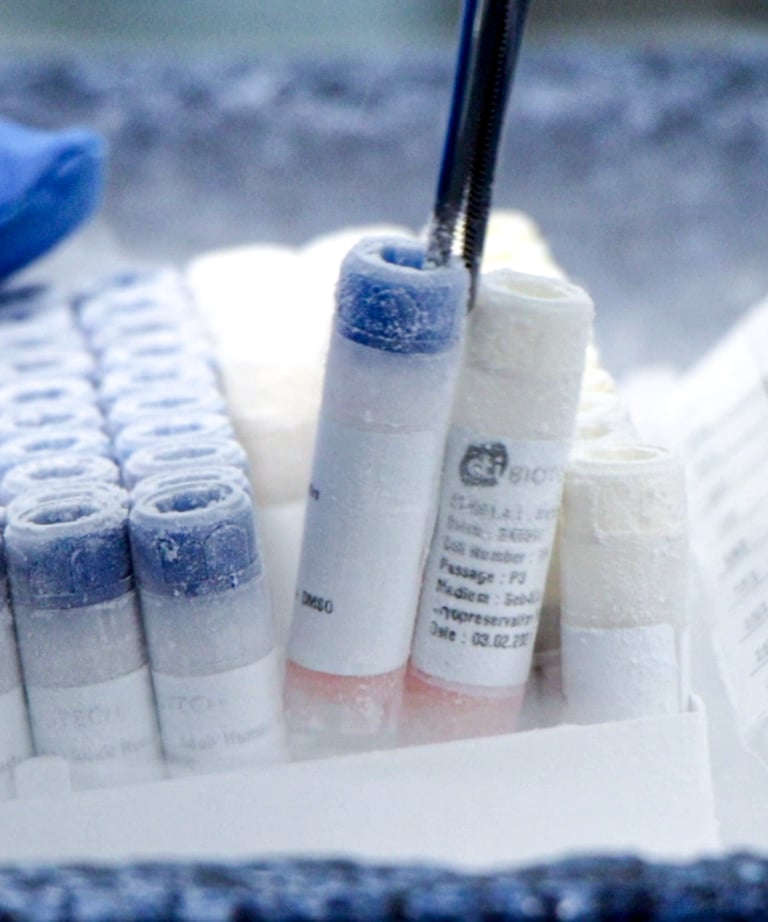
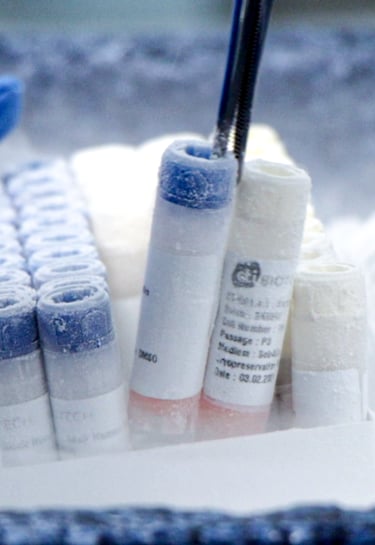
Pharmaceutical innovation
and testing
Cells and tissues testing for pharmaceuticals, vaccines and medical devices
Buy cells and tissues
We can provide you with human cells and tissues for research and testing in your own lab








Certifications
We operate to the highest standards. We are fully authorized to work with human tissues, certified ISO 9001 : 2015 and recognized as a fast-growing company "PERL" by the French government.






Get In Touch
Address
CTIBIOTECH
Bat A16, 5 avenue Lionel Terray
69330 Meyzieu
Lyon, France
Subscribe to our newsletter

